Did you know that liver cancer is the 4th most common cancer in the Philippines (and that Billiary Tract cancer is actually a sub type )?
Moreover, majority of liver cancers are Hepatocellular Carcinomas. In fact, Biliary Tract Cancers are rarer in which malignant (cancer) cells form in the bile ducts.
These are just some information we learned at the recent TOPAZ-1 study Philippine launch with HCPs and other media at the Edsa Shangri-La Hotel last week, upon the invitation of AstraZeneca, a global science-led biopharmaceutical company.

TOPAZ-1: First Global Phase III study for an Immuno-Oncology (IO) combination in first-line advanced BTC
AstraZeneca announced the breakthrough results from the TOPAZ-1 Phase III trial for liver cancer research, more specifically for Biliary Tract Cancer . It showed that Durvalumab, in combination with standard-of-care chemotherapy, demonstrated a clinically meaningful and durable overall survival (OS) benefit as a treatment for patients with advanced biliary tract cancer (BTC)*.
Do-Youn Oh, MD, PhD, Professor, Division of Medical Oncology, Department of Internal Medicine at Seoul National University Hospital and Seoul National University College of Medicine, and principal investigator in the TOPAZ-1 Phase III trial, said:
“After minimal progress for more than a decade in advanced biliary tract cancer, the TOPAZ-1 results are a tremendous advance for our patients, showing a clear survival benefit for Durvalumab added to chemotherapy compared to standard of care with a remarkable safety profile. This combination will provide a desperately needed and potentially practice-changing new treatment option in a setting where the current prognosis is devastating.”



Susan Galbraith, Executive Vice President, Oncology R&D, AstraZeneca, said: “The results from the TOPAZ-1 trial challenge treatment expectations in advanced biliary tract cancer and provide compelling evidence that longer-term survival is possible. Overall survival improves over time with an estimated one in four patients on Durvalumab plus chemotherapy alive at two years compared to one in ten on chemotherapy alone. This is a potential new standard of care for patients in this setting and we remain committed to making advances in gastrointestinal cancers with high unmet need.”
In a predefined interim analysis, patients treated with Durvalumab in combination with standard-of-care chemotherapy experienced a 20% reduction in the risk of death versus chemotherapy alone (based on a hazard ratio [HR] of 0.80; 95% confidence interval [CI], 0.66-0.97; 2-sided p=0.021). Median OS was 12.8 months versus 11.5 for chemotherapy. An estimated 25% of patients were still alive at two years versus 10% for chemotherapy*.
Results also showed a 25% reduction in the risk of disease progression or death with Durvalumab plus chemotherapy (HR, 0.75; 95% CI, 0.64-0.89; 2-sided p=0.001). Median PFS was 7.2 months for the combination versus 5.7 for chemotherapy. Patients treated with Imfinzi plus chemotherapy achieved an objective response rate (ORR) of 26.7% versus an ORR of 18.7% for patients treated with chemotherapy alone*.
Summary of Efficacy Results
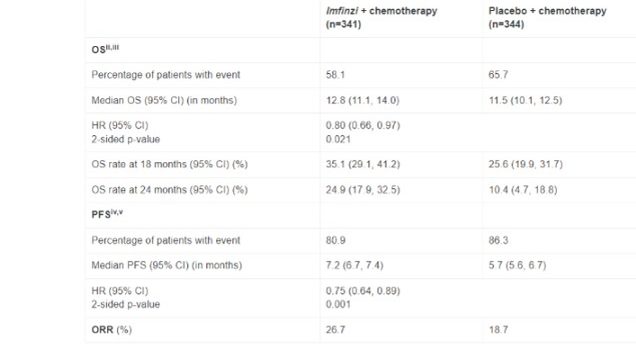
- Analysis was done at 62% maturity in OS data.
- Investigator-assessed OS data cut-off date was 11 August 2021.
- Median follow-up in censored patients at DCO: 13.7 months (range 0.4-27.2) for Imfinzi plus chemotherapy, 12.6 months (range 0.7-26.0) for chemotherapy alone.
- Investigator-assessed PFS data cut-off date was 11 August 2021.
- Median follow-up in censored patients at DCO: 9.2 months (range 0.0-24.0) for Imfinzi plus chemotherapy, 6.9 months (range 0.0-20.4) for chemotherapy alone.
Durvalumab plus chemotherapy did not increase the discontinuation rate due to adverse events (AEs) compared to chemotherapy alone. Grade 3 or 4 treatment-related AEs were experienced by 62.7% of patients treated with Durvalumab and chemotherapy, and by 64.9% of patients receiving chemotherapy alone. Treatment-related AEs led to discontinuation in 8.9% of patients treated with the Durvalumab combination versus 11.4% of patients receiving chemotherapy*.

AstraZeneca in Oncology
According to Lotis Ramin, AstraZeneca Philippines Country President, “We are leading a revolution in oncology to redefine cancer care, with the ambition to provide cure through our life-changing medicines alongside solutions and partnerships that allow early detection, diagnosis, and equitable care for Filipino patients with cancer.”
She furthers, “It is through persistent innovation, collaboration and commitment to patients that AstraZeneca has built one of the most diverse portfolios and pipelines in the industry, with the potential to catalyse changes in the practice of medicine and transform the patient experience.”
Disease Burden and Treatment Options
BTC is a group of rare and aggressive gastrointestinal (GI) cancers that form in the cells of the bile ducts (cholangiocarcinoma), gallbladder or ampulla of Vater (where the bile duct and pancreatic duct connect to the small intestine).**,***
Approximately 80% of patients with BTC will be diagnosed with advanced disease **** with low 5-year survival rate across all major subtypes of BTC *****.
Biliary tract cancer is relatively uncommon, accounting for less than 1% of all human cancers. It is most commonly diagnosed in people between the ages of 60 and 70 years and affects slightly more men than women ****.
BTC is a major sub-type of liver cancer, which is the fourth most common cancer type in the Philippines and the third leading cause of cancer death in country ****.
Treatment for biliary tract cancer depends on the size, location, and stage of the tumour ***. Current treatment options include surgery, chemotherapy, and radiation therapy.
Immunotherapy and target therapy are upcoming treatment options for patients with BTC ******.
Immuno-oncology, or cancer immunotherapy, has been successful in the targeted treatment of cancer, but recent advances in understanding of the immune system are informing the development of the next wave of therapies with the potential to make an even greater impact for patients across all stages of disease.

References:
* Do-Youn Oh, et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evidence. 2022;1(8). DOI: 10.1056/EVIDoa2200015.
** Marcano-Bonilla L, et al. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. CCO. 2016;5(5).
*** ESMO. What is Biliary Tract Cancer. Available at: https://www.esmo.org/content/download/266801/5310983/1/EN-Biliary-Tract-Cancer-Guide-for-Patients.pdf. Accessed September 2022.
**** Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2016;27(suppl 5):28-37.
***** Global cancer statistics 2022: Philippine Fact Sheet. https://gco.iarc.who.int/media/globocan/factsheets/populations/608-philippines-fact-sheet.pdf
****** NCCN Guideline For Biliary Tract Cancers v3.2023. https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf
Disclaimer: Durvalumab in combination with Gem-Cis has now been approved for use in Biliary Tract Cancer, In December 23. Durvalumab is also approved for the following indications: 1. Extensive Stage -Small Cell Carcinoma, ES-SCLC. 2. Locally Advanced Unrespectable Stage 3 Non Small Cell Lung Cancer 3. Advanced Biliary Tract Cancer. Durvalumab is a prescription medicine. Please consult your doctor for further information and advice on the medication. For suspected adverse drug reaction, please report to the Food and Drug Administration (FDA) at www.fda.gov.ph or to AstraZeneca at https://contactazmedical.astrazeneca.com. Patients should seek medical attention immediately at the first sign of any adverse drug reaction

0 Comments